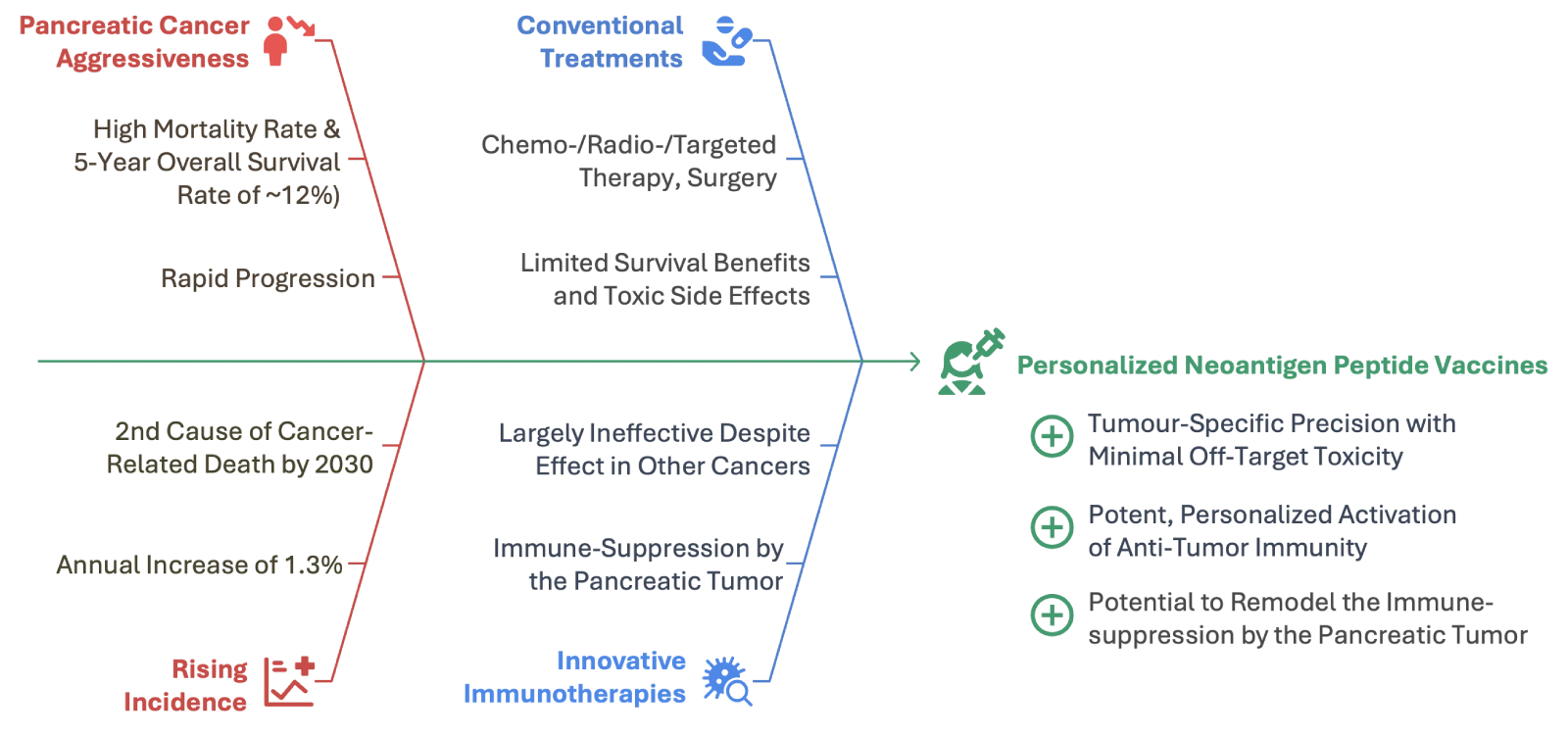
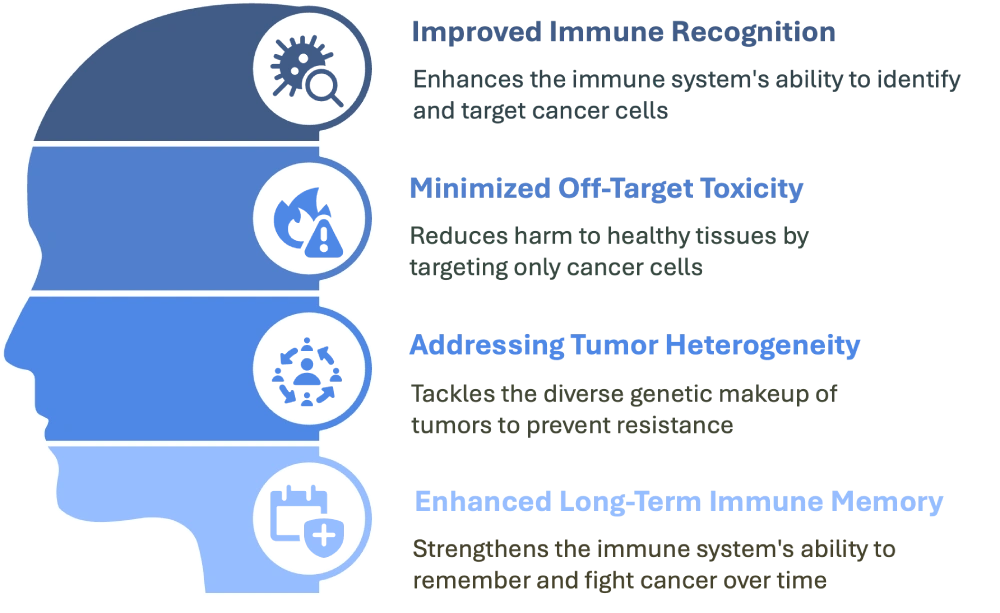
Product
At NeOncoFRAx, we believe that every tumor carries a unique genetic fingerprint—one that can be decoded to help the immune system distinguish friend from foe. By analyzing the DNA of a patient’s tumor, we identify specific mutations that generate neoantigens: protein fragments that exist only in cancer cells. Our vaccines utilize these tumor-specific markers to train the immune system to recognize and eliminate malignant cells, while sparing healthy tissue.
Our vaccines present these neoantigens to the immune system, priming T cells to recognize and destroy the cancer. This precision approach offers the potential for greater efficacy and lower toxicity compared to conventional immunotherapies.