Tumor and Blood Collection
The process begins during routine surgery, when a section of the tumor and a blood sample are collected for molecular and immunological analysis.
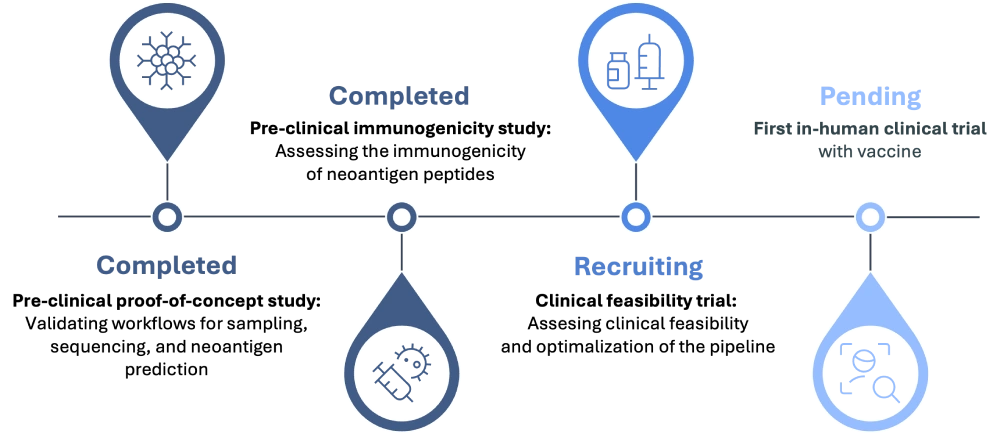
At NeOncoFRAx, our pipeline transforms scientific discovery into clinical care—one patient at a time. Through a fully integrated academic collaboration with Erasmus MC, we bring together surgeons, immunologists, bioinformaticians, chemists, and clinical researchers to design and deliver personalized cancer vaccines within a matter of months. This end-to-end approach ensures scientific rigor, fast decision-making, and seamless translation from bench to bedside.
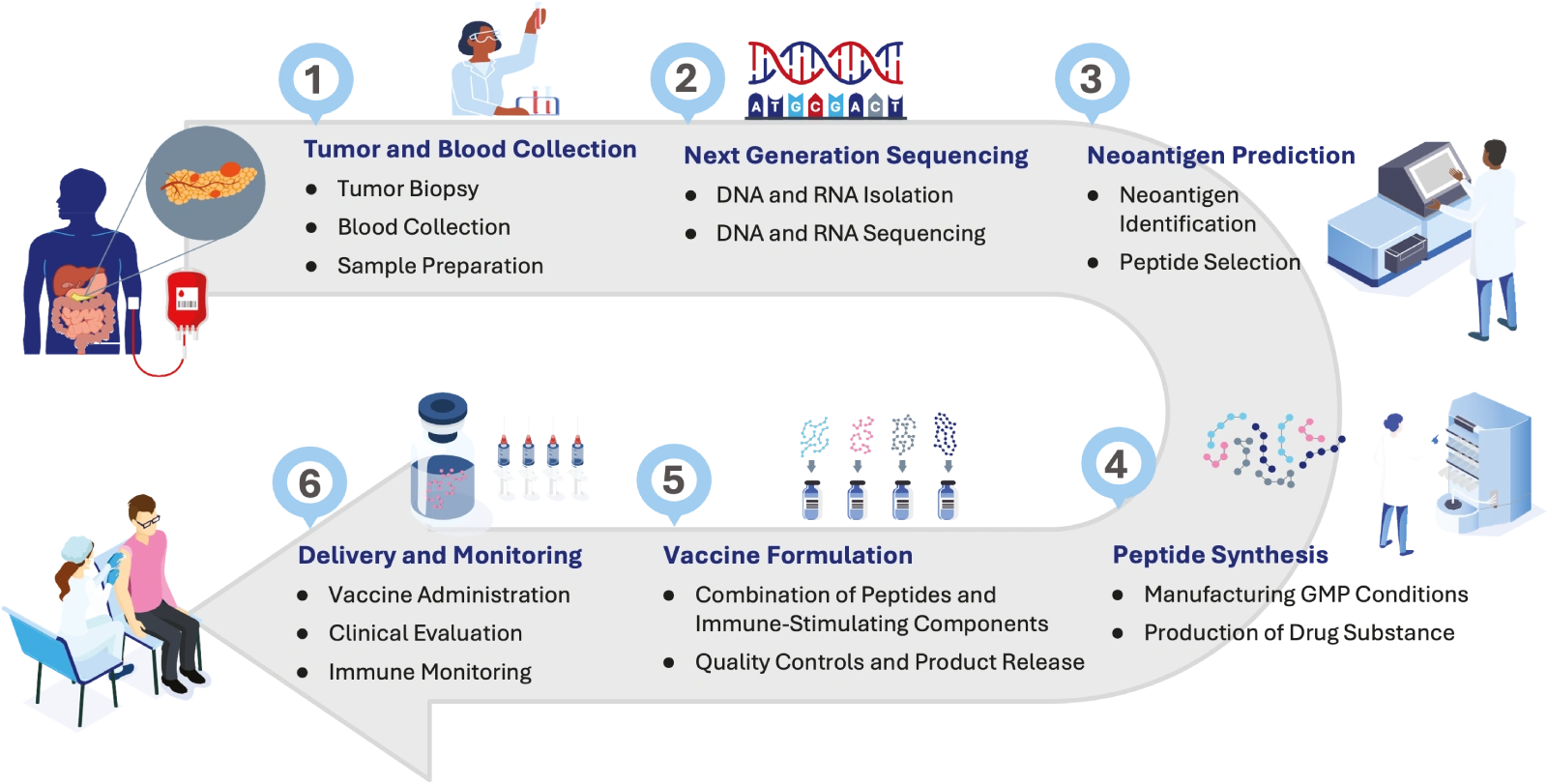
The process begins during routine surgery, when a section of the tumor and a blood sample are collected for molecular and immunological analysis.
These samples undergo next-generation sequencing to identify mutations unique to the cancer.
Our bioinformatics team analyzes sequence data using a multi-layered algorithm. Peptides are scored and prioritized based on MHC binding affinity, transcript expression, variant frequency, and predicted immunogenicity.
The top-ranked neoantigens are synthesized into peptides through academic or partnered facilities under GMP-adjacent quality controls. (Note: In-house synthesis is a key ambition in progress.)
Peptides are combined with clinically validated immune-stimulating components, which may include dendritic cell loading, to create a patient-specific vaccine batch.
Patients receive their personalized vaccine under the supervision of a medical professional. Immune responses are tracked longitudinally through blood-based profiling to assess safety, feasibility, and activation of tumor-reactive T cells.